The allowed values of l depend on the value of n and can range from 0 to n − 1: Equation 2.5.2 l = 0, 1, 2,…, n − 1. For example, if n = 1, l can be only 0; if n = 2, l can be 0 or 1; and so forth. For a given atom, all wave functions that have the same values of both n and l form a subshell.
SOLVED: Rank the following orbitals in terms of their energies: 4s 2s 3s 1s 3p 2p
The order of the electron orbital power stages, starting from least to best, is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3-d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. since electrons all have the same price, they live as some distance away as feasible because of repulsion.

Source Image: reddit.com
Download Image
Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer Within the same energy level, the order of increasing energy for the orbitals
Source Image: quora.com
Download Image
⏩SOLVED:Rank the following orbitals in the H atom in order of… | Numerade Different from the predictions of Newtonian mechanics, which allows any energy to be possible, Bohr described the electron orbits (now called orbitals) as having specific energies. Rank the following electron energy states according to their electron energies.

Source Image: byjus.com
Download Image
Rank The Following Orbitals In Terms Of Their Energies
Different from the predictions of Newtonian mechanics, which allows any energy to be possible, Bohr described the electron orbits (now called orbitals) as having specific energies. Rank the following electron energy states according to their electron energies. Chemistry Chemistry questions and answers Rank the following orbitals in terms of their energies. 5p 6d 4f 7s 5d 5f This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Rank the following orbitals in terms of their energies. 5p 6d 4f 7s 5d 5f
Quantum Numbers (Principal, Azimuthal, Magnetic and Spin) – Definition, Detailed Explanation, Videos and FAQs of Quantum Numbers.
Apr 21, 2022The ground-state electron configurations of the elements are listed in Table 9.9.9B. 1 9.9.9 B. 1. The “exceptions” to the simple mnemonic noted in general chemistry texts are partly a consequence of the inadequacy of a “one-orbital order-fits-all” model. For example, copper has an electron configuration of [Ar]4s 1 d 10. Chemistry Chapter 6 SG Flashcards | Quizlet

Source Image: quizlet.com
Download Image
MO Diagrams of Main Group Elements [More Practice] – Wize University Chemistry Textbook | Wizeprep Apr 21, 2022The ground-state electron configurations of the elements are listed in Table 9.9.9B. 1 9.9.9 B. 1. The “exceptions” to the simple mnemonic noted in general chemistry texts are partly a consequence of the inadequacy of a “one-orbital order-fits-all” model. For example, copper has an electron configuration of [Ar]4s 1 d 10.
![MO Diagrams of Main Group Elements [More Practice] - Wize University Chemistry Textbook | Wizeprep](https://d3rw207pwvlq3a.cloudfront.net/attachments/000/075/685/images/img_poster.0000000.jpg?1615771517)
Source Image: wizeprep.com
Download Image
SOLVED: Rank the following orbitals in terms of their energies: 4s 2s 3s 1s 3p 2p The allowed values of l depend on the value of n and can range from 0 to n − 1: Equation 2.5.2 l = 0, 1, 2,…, n − 1. For example, if n = 1, l can be only 0; if n = 2, l can be 0 or 1; and so forth. For a given atom, all wave functions that have the same values of both n and l form a subshell.

Source Image: numerade.com
Download Image
⏩SOLVED:Rank the following orbitals in the H atom in order of… | Numerade Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer Within the same energy level, the order of increasing energy for the orbitals
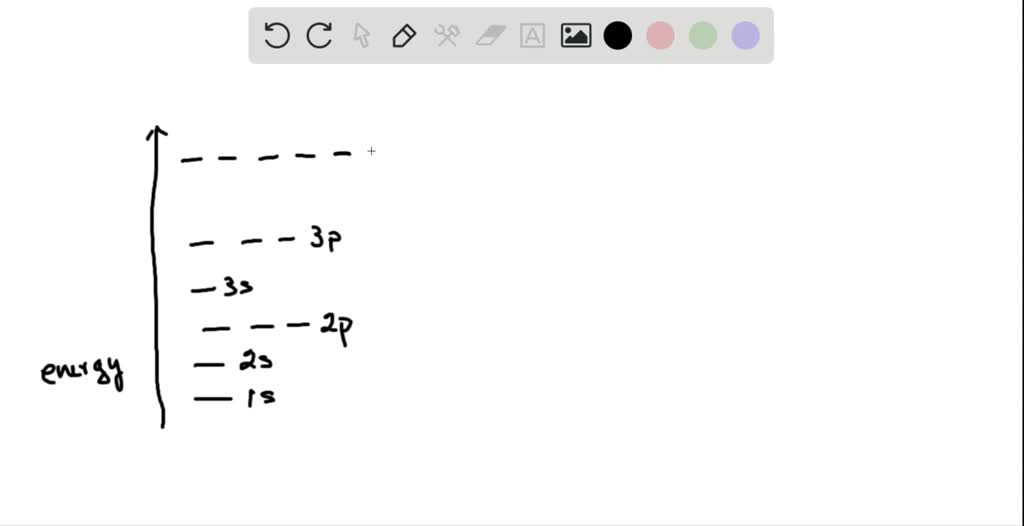
Source Image: numerade.com
Download Image
SOLVED: Rank the following orbitals in terms of their energies: 4s 2s 3s 1s 3p 2p The orbitals are s, p, d, and f. How many electrons can each orbital hold? orbital type. Describes the shape of the orbital (s, p, d, f) specific orbital. Meaning, it determines how many orbitals there are of a type per energy level. spin and can only be +1/2 or -1/2. Also, no two electrons in an atom can have the same four quantum numbers as

Source Image: numerade.com
Download Image
Rank The Following Orbitals in Terms of Their Energies | TikTok Different from the predictions of Newtonian mechanics, which allows any energy to be possible, Bohr described the electron orbits (now called orbitals) as having specific energies. Rank the following electron energy states according to their electron energies.

Source Image: tiktok.com
Download Image
⏩SOLVED:Given the orbital filling diagrams on the left for the… | Numerade Chemistry Chemistry questions and answers Rank the following orbitals in terms of their energies. 5p 6d 4f 7s 5d 5f This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Rank the following orbitals in terms of their energies. 5p 6d 4f 7s 5d 5f
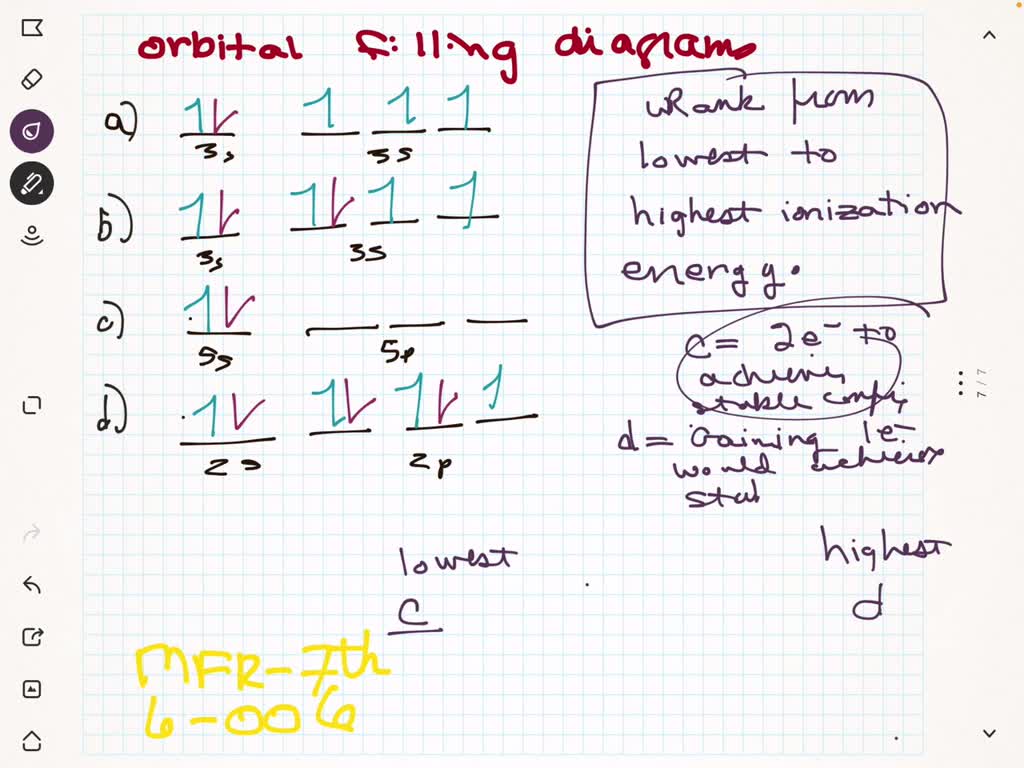
Source Image: numerade.com
Download Image
MO Diagrams of Main Group Elements [More Practice] – Wize University Chemistry Textbook | Wizeprep
⏩SOLVED:Given the orbital filling diagrams on the left for the… | Numerade The order of the electron orbital power stages, starting from least to best, is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3-d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. since electrons all have the same price, they live as some distance away as feasible because of repulsion.
⏩SOLVED:Rank the following orbitals in the H atom in order of… | Numerade Rank The Following Orbitals in Terms of Their Energies | TikTok The orbitals are s, p, d, and f. How many electrons can each orbital hold? orbital type. Describes the shape of the orbital (s, p, d, f) specific orbital. Meaning, it determines how many orbitals there are of a type per energy level. spin and can only be +1/2 or -1/2. Also, no two electrons in an atom can have the same four quantum numbers as