Chemistry questions and answers Which of the following will be the strongest acid? A) PH3 B) SIHA C) HAS D) BH3 Tap here or pull up for additional resources Which of the following will be the strongest acid? A) H2O B) HzTe C) H2S D) H Se This problem has been solved!
Which amongst the following is the strongest acid?
Jul 12, 2023Strong Bases. Strong bases are completely ionized in solution. Table 16.4.1 16.4. 1 includes some common strong bases. For example, KOH KOH dissolves in water in the reaction. KOH → K+ +OH− KOH → K + + OH −. Relative to the number of strong acids, there are fewer number of strong bases and most are alkali hydroxides.
Source Image: www.sarthaks.com
Download Image
Jul 7, 2023The strongest acids are at the bottom left, and the strongest bases are at the top right. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. In an acid-base reaction, the proton always reacts with the stronger base.
Source Image: www.chegg.com
Download Image
Solved Choose the compound that is the strongest acid. O | Chegg.com Nov 17, 2023the strongest acid Known: The hydrohelium Cation. The stornger acid, the weaker the covalent bond to a hydrogen atom. So the strongest acid possible is the molecule with the weakest bond. That is the hydrohelium (1+) cation, \(\ceHeH^+\), which is a positively charged ion formed by the reaction of a proton with a helium atom in the gas phase.
Source Image: www.chegg.com
Download Image
Which Of The Following Will Be The Strongest Acid
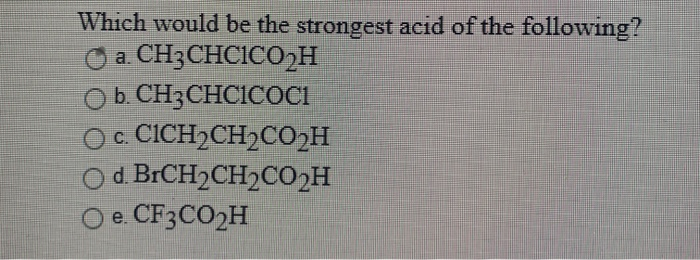
Nov 17, 2023the strongest acid Known: The hydrohelium Cation. The stornger acid, the weaker the covalent bond to a hydrogen atom. So the strongest acid possible is the molecule with the weakest bond. That is the hydrohelium (1+) cation, \(\ceHeH^+\), which is a positively charged ion formed by the reaction of a proton with a helium atom in the gas phase. A CH3COOH B CH2ClCOOH C CHCl2COOH D CCl3COOH Solution Verified by Toppr Electron withdrawing groups increase the acidity of carboxylic acid as they stablize the conjugate base, i.e., carboxylate ion. Chloride ion is an electron withdrawing group.
Solved 9. Which of the following is the strongest acid? | Chegg.com
By Anne Marie Helmenstine, Ph.D. Updated on May 05, 2019 What is the world’s strongest acid? It’s probably not one you’d guess. None of the strong acids traditionally listed in a chemistry text holds the title of World’s Strongest Acid. Solved Which of the following is the strongest acid? | Chegg.com
Source Image: www.chegg.com
Download Image
List of Common Strong and Weak Acids By Anne Marie Helmenstine, Ph.D. Updated on May 05, 2019 What is the world’s strongest acid? It’s probably not one you’d guess. None of the strong acids traditionally listed in a chemistry text holds the title of World’s Strongest Acid.
:max_bytes(150000):strip_icc()/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)
Source Image: www.thoughtco.com
Download Image
Which amongst the following is the strongest acid? Chemistry questions and answers Which of the following will be the strongest acid? A) PH3 B) SIHA C) HAS D) BH3 Tap here or pull up for additional resources Which of the following will be the strongest acid? A) H2O B) HzTe C) H2S D) H Se This problem has been solved!

Source Image: m.youtube.com
Download Image
Solved Choose the compound that is the strongest acid. O | Chegg.com Jul 7, 2023The strongest acids are at the bottom left, and the strongest bases are at the top right. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. In an acid-base reaction, the proton always reacts with the stronger base.

Source Image: www.chegg.com
Download Image
The strongest acid from the following is – Sarthaks eConnect | Largest Online Education Community A total of seven acids are widely regarded as “strong” acids in the field of chemistry. The list of strong acids is provided below. Hydrochloric acid (denoted by the chemical formula HCl) Hydrobromic acid (denoted by the chemical formula HBr) Hydroiodic acid or hydriodic acid (denoted by the chemical formula HI) Sulfuric acid (denoted by
Source Image: www.sarthaks.com
Download Image
The strongest acid amongst the following compounds is Nov 17, 2023the strongest acid Known: The hydrohelium Cation. The stornger acid, the weaker the covalent bond to a hydrogen atom. So the strongest acid possible is the molecule with the weakest bond. That is the hydrohelium (1+) cation, \(\ceHeH^+\), which is a positively charged ion formed by the reaction of a proton with a helium atom in the gas phase.

Source Image: byjus.com
Download Image
Solved Which would be the strongest acid of the following? a | Chegg.com A CH3COOH B CH2ClCOOH C CHCl2COOH D CCl3COOH Solution Verified by Toppr Electron withdrawing groups increase the acidity of carboxylic acid as they stablize the conjugate base, i.e., carboxylate ion. Chloride ion is an electron withdrawing group.
Source Image: www.chegg.com
Download Image
List of Common Strong and Weak Acids
Solved Which would be the strongest acid of the following? a | Chegg.com Jul 12, 2023Strong Bases. Strong bases are completely ionized in solution. Table 16.4.1 16.4. 1 includes some common strong bases. For example, KOH KOH dissolves in water in the reaction. KOH → K+ +OH− KOH → K + + OH −. Relative to the number of strong acids, there are fewer number of strong bases and most are alkali hydroxides.
Solved Choose the compound that is the strongest acid. O | Chegg.com The strongest acid amongst the following compounds is A total of seven acids are widely regarded as “strong” acids in the field of chemistry. The list of strong acids is provided below. Hydrochloric acid (denoted by the chemical formula HCl) Hydrobromic acid (denoted by the chemical formula HBr) Hydroiodic acid or hydriodic acid (denoted by the chemical formula HI) Sulfuric acid (denoted by